BioDuro’s in vitro biology team has developed a comprehensive profiling platform using Halda's patented molecule (WO 2025/085738 A1) as a reference standard. Our platform spans from mechanism-of-action validation and activity assessment to specificity analysis, enabling a systematic evaluation through rigorously controlled, parallel experiments.

BioDuro has established and validated a novel LC-HRMS workflow for fast, global metabolite profiling and confident identification of peptide therapeutics regardless of their molecular ions, formation pathways, and fragmentation behaviors.

BioDuro’s peptide chemistry team has advanced the development of DNA-encoded peptide libraries (DELs) by establishing robust, high-throughput synthetic platforms that enable the construction of diverse, macrocyclic peptide libraries with enhanced chemical flexibility.

This article outlines an integrated ADME strategy for ADCs from lead optimization to development, based on our experiences, recommendations from regulatory guidelines, and industry position papers. A decision tree is shared to guide payload bioanalysis, PK and DDI evaluation, and biotransformation studies.

BioDuro's biology team developed a comprehensive assay package for in vitro pharmacological evaluation of STAT6 PROTACs using Kymera I-1 from Kymera patent and AK-1690 from Dr. Shaomeng Wang's team at the University of Michigan as reference compounds.

BioDuro's DMPK team developed and validated a 7-in-1 Cocktail Assay, which scientifically consolidates all DDI risk assessments into a single incubation and rapid LC-MS/MS injection, dramatically reducing screening time and cost while maintaining the high data quality required for regulatory confidence.

By establishing a validated working range for the 96-well format, the study defines a robust and reproducible operating framework for hepatocyte stability assays. The optimized conditions prove more efficient without compromising biological relevance, maintaining metabolic integrity and data continuity and allowing larger compound sets to be assessed.

BioDuro provides versatile flow chemistry solutions to support efficient route development and scale-up. The flow chemistry platform enables safe control of challenging reaction conditions while improving reproducibility and process robustness for early development and manufacturing needs.

This case study demonstrates BioDuro’s bioconjugation capability to efficiently transition complex ADC processes from bench scale to Gram scale within a rapid 7-day timeline. By meticulously controlling the Trastuzumab-based conjugation, we achieved industry-leading quality metrics: precise DAR and ultra-low free drug clearance (less than 0.09% mol/mol). Crucially, post-process functional validation confirmed that the ADC product fully preserved the binding affinity of the antibody and the cytotoxic potency of the payload, ensuring translational success in preclinical studies.

In the intricate journey of drug development, a compound's journey through the body is fundamentally governed by its lipophilicity. This crucial property, quantified by LogP (intrinsic lipophilicity of the neutral form) and LogD (lipophilicity at a specific pH, accounting for ionization), dictates a molecule's ability to cross biological membranes, reach its target, and ultimately exert its therapeutic effect.

Breast cancer is not a single disease but a collection of biologically distinct subtypes, each with unique molecular features and treatment challenges. Emerging therapies — including immunotherapies, antibody–drug conjugates (ADCs), and targeted drugs such as VEGF, EGFR, PARP, PI3K/Akt/mTOR, and CDK4/6 inhibitors — are offering new hope for patients.

BioDuro offers tailored decision frameworks and fit-for-purpose ADME strategies for ADCs—from early in vitro profiling to regulatory studies—enabling data-driven decisions that accelerate ADC drug development.

At the preclinical level, BioDuro's DIO (diet-induced obesity) rodent models, combined with high-resolution phenotyping tools, provide a translational bridge to accelerate anti-obesity drug development.

This case study details the validation of vesicle-based substrate assays using human-derived inside-out membrane vesicles expressing BSEP and MRP1–MRP4. This validated model provides a critical in vitro tool for understanding drug-transporter interactions, enabling safer compound selection and early de-risking.

Amorphous Solid Dispersion (ASD) technology has proven effective in enhancing the solubility and bioavailability of poorly soluble APIs. The proposed ASD screening platform offers several advantages for new chemical entities (NCEs) in early discovery.

At BioDuro-Sundia, we leverage decades of experience and expertise in peptide chemistry to deliver high-quality peptides, from milligram to gram scales.

The results for DS-8201a ADC indicated a slow elimination with a T1/2 of 8.5 days, relatively low Vss and CLin mice, which are consistent with literature' values.This also demonstrated that 1 mg ADC and serial bleeding of each mouse are sufficient to perform a high-quality mouse PK profile.

In this study, we analyzed and showed changes in T-DM1 DAR distribution in plasma from HER2-positive gastric tumor-bearing C.B-17 SCID mice using UPLC/high-resolution mass spectrometry (HRMS).

At BioDuro, we are ready to drive innovation in this rapidly growing GLP-1 market with our comprehensive expertise and integrated solutions.

We offer a fragment-to-lead workflow combining high-throughput screening, structural insights, and virtual library screening to accelerate hit identification and optimization.

Our peptide team at BioDuro successfully completed a complex 42-step liquid-phase synthesis of MK-0616, a PCSK9-targeting reference peptide, in 7 weeks and delivered 1 gram of the final compound.

This novel High-Throughput Peptide Library Platform represents a significant advancement, effectively addressing critical bottlenecks in peptide drug discovery.

BioDuro leverages its formulation development expertise, extensive experience in solubilization technologies, and its superb DMPK team and facilities to provide rapid and innovative solutions to enhance the bioavailability of poorly soluble drug candidates.
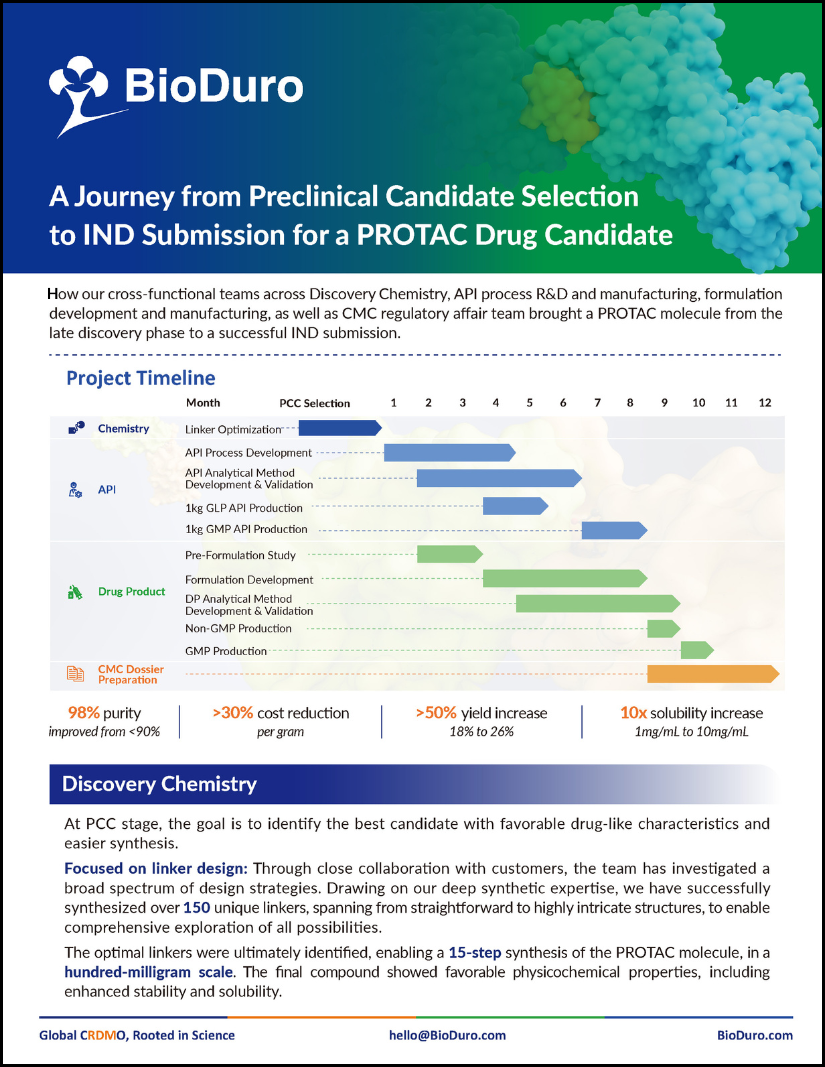
BioDuro cross-functional teams brought a complex PROTAC molecule from the late discovery phase to a successful IND submission. Download this flyer to see the breadth of our services.

We provide our biotech and pharmaceutical partners with fully integrated services to support their efforts from target identification through to commercial drug product manufacturing. Download this flyer to see the breadth of our services.

Our amorphous solid dispersion (ASD) screening platform, which we call our Solution Engine, combines in-silico, in-vitro and in-vivo studies and enables cost-effective use of API during early formulation development to enhance bioavailability.
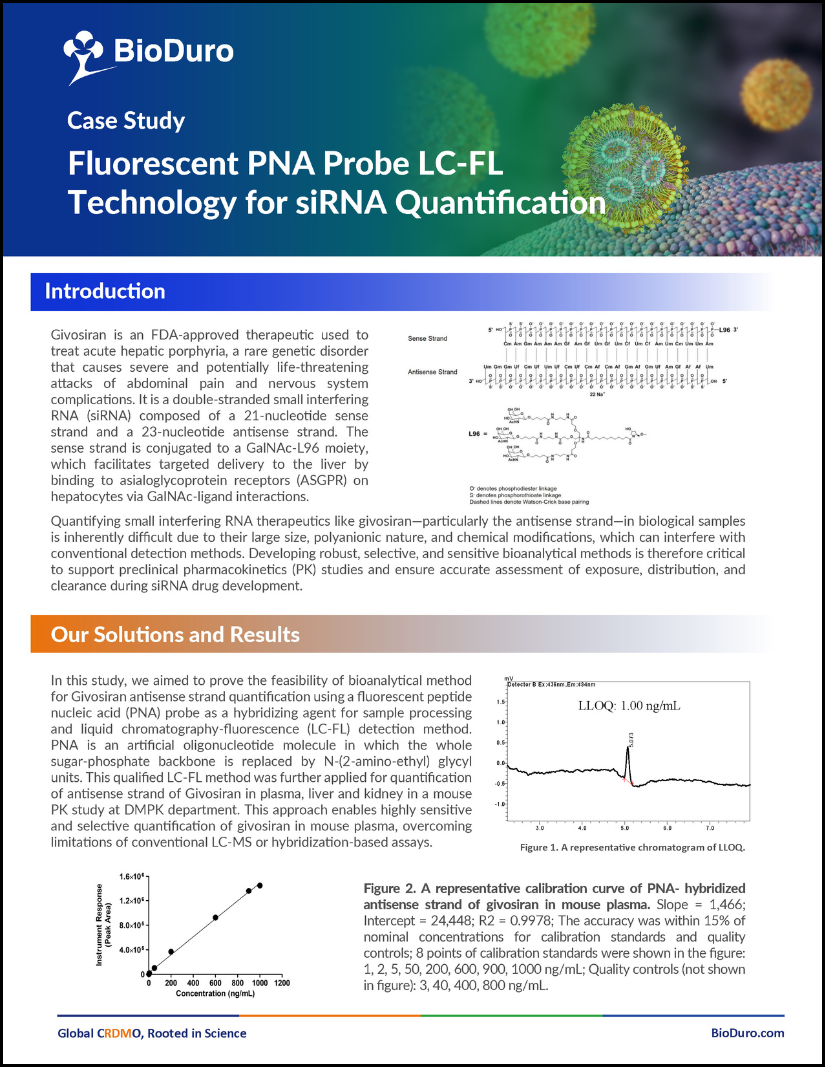
A fluorescent PNA probe coupled liquid chromatography-fluorescence (LC-FL) method for quantification of givosiran has been established and successfully applied to investigate givosiran PK and tissue distribution in mice.

BioDuro's peptide chemistry team successfully completed a challenging 37-step solid-phase synthesis of I-66 acetate, a complex macrocyclic peptide, in just 6 weeks, delivering 50 mg of the final compound.

Learn about BioDuro's scalable and phase-appropriate route scouting and optimization strategies, which include dose range finding (DRF), GMP Enabling and other process R&D service capabilities.

Our DMPK department completed a 7-day study to analyze the drug-to-antibody ratio (DAR) distribution of an ADC in HER2-positive tumor-bearing mouse plasma using UPLC/HRMS.
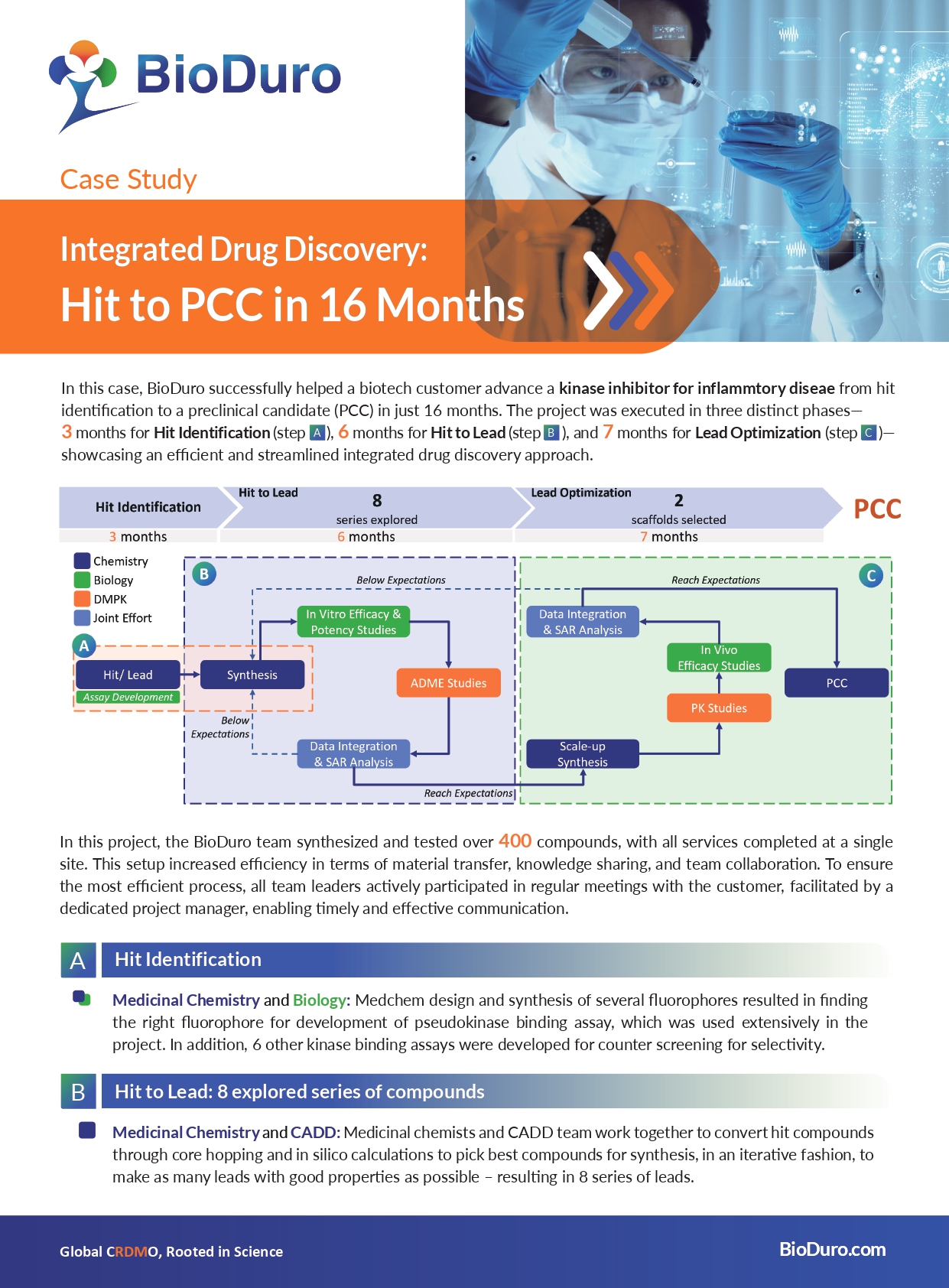
Our uniquely integrated drug discovery platform advanced a biotech customer's kinase inhibitor for inflammatory disease from hit identification to a preclinical candidate (PCC) in just 16 months.

View our recent IND Enabling Services case study of an 11 month timeline to IND ready. Learn about our API process development and drug product formulation alongside both non-GMP and GMP production activities, and the final CTD documents.

Designed to support ADC drug development from target identification to PCC, BioDuro excels at ADC-related custom synthesis as well as bioconjugation services and in-vitro/in-vivo efficacy evaluation.

Comprehensive and readily available drug metabolism identification, profiling, stability and inhibition services with extensive capabilities in Cytochrome P450 (CYP) and UDP-glucuronosyltransferases (UGT).

Our analytical development team is well-versed to facilitate the development of the analytical methods and testing of prototype formulations, raw materials, and drug products spanning from Pre-IND early phase to late registration.

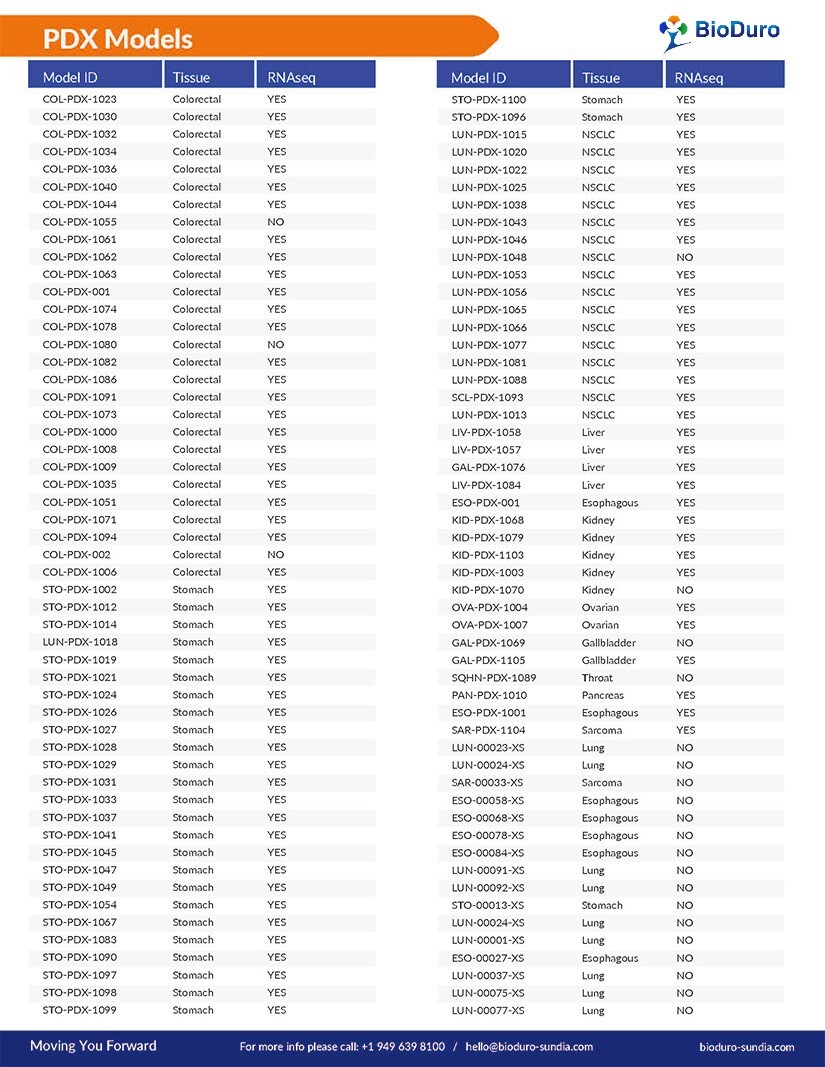
A full list of all our available CDX Models includes: bladder cancer, blood cancer, brain cancer, breast cancer, cervix cancer, choriocarcinoma, colorectal cancer, esophageal cancer, fibrosarcoma, gastric cancer, kidney cancer, and more.

Learn about our automated, flexible tailored solutions to store and manage compounds in both liquid and solid formats. Our compound management platform includes the advanced Hamilton Liquid Handling System and end-to-end Mosaic System, providing accurate and efficient oversight of our compound inventory.

Discover our integrated drug discovery solutions with a wide range of cancer research models, immuno-oncology techniques, and comprehensive cellular and biomarker analyses. Our services include drug target discovery, potency and efficacy evaluation, and access to the LOCUS Queryable Database to identify oncology models.

Partner with us for your drug discovery project needs. We provide a wide range of services, including custom assays, stable cell line generation, SPR and BLI services, and fragment library screening, with broad expertise in therapeutic disease areas and target class.

Partner with our experienced and specialized team to solve complex chemistry problems. As a service-oriented business, we offer long-term partnerships and tailored solutions. We will help you to achieve your chemistry goals.

Comprehensive in-vitro and in-vivo services to identify a pre-clinical drug candidate’s potential toxicities or adverse effects.

Fast and reliable DMPK services covering in vitro and in vivo assays to evaluate ADME and PK properties, and subsequent preclinical services for a wide range of modalities.
Our comprehensive range of services, from early drug discovery to commercial API production, includes drug substance development, process R&D, scale-up synthesis, and integrated IND enabling services. Our expert team is dedicated to ensuring quality and efficiency to help you bring new drugs to market quickly and effectively.

Offering a diverse and efficient fragment library with novel scaffolds and important pharmacophores, excluding functional groups with known structural alerts, for drug discovery projects. Learn more about our screening and hit follow-up options.

We leverage decades of experience and expertise in peptide chemistry to deliver high-quality peptide, from milligram to gram scale. Our state-of-art facilities, including a dedicated 1500 ㎡ peptide synthesis center in Beijing, are equipped with top-class instruments and supported by a team of 100+ experienced chemists.

BioDuro in vitro and in vivo drug discovery services address preclinical research needs in the development of pharmaceuticals to treat immune, autoimmune, and inflammatory diseases.

Fast and flexible in vivo PK services with 10 species of rodents, small and large animals, and a range of advanced administration and bioanalysis techniques.